Fe2S3 + O2 / PPT - IOD PowerPoint Presentation, free download - ID:6634718 / In the balanced reaction shown the mole ratio of fe2s3 to o2 is.
Mol of o2 → mol of h2o → grams of h2o. Application for completing products and balancing . How many moles of oxygen are produced by the decomposition of six moles of. Solution for consider the reaction 2 fe2s3 (s) + 9 o2 yields 2 fe2o3 (s) + 6 so2 (g) the reaction is carried out and kept at 1.00 atm pressure and 215 c… In the balanced reaction shown the mole ratio of fe2s3 to o2 is.

Mol of o2 → mol of h2o → grams of h2o.
Complete the reaction equation by entering the … How many moles of oxygen are produced by the decomposition of six moles of. Mol of o2 → mol of h2o → grams of h2o. No elastic tensor calculated for this material, so elastic energies not avaialable. Li3(fe2s3)2 crystallizes in the monoclinic c2/m space group. Application for completing products and balancing . Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28. Substrate material, substrate orientation, film . There are two inequivalent li1+ sites. Solved and balanced chemical equation 2 fe2s3 + 9 o2 → 6 so2 + 2 fe2o3 with completed products. To determine the number of moles of oxygen needed to react with 3.2 moles of c4h10. 2 fe + 3 s → fe2s3. 1.5fe2s3 + 6.5o2(g) = fe3o4 + 4.5so2(g).
To determine the number of moles of oxygen needed to react with 3.2 moles of c4h10. No elastic tensor calculated for this material, so elastic energies not avaialable. 2 fe + 3 s → fe2s3. Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28. Substrate material, substrate orientation, film .
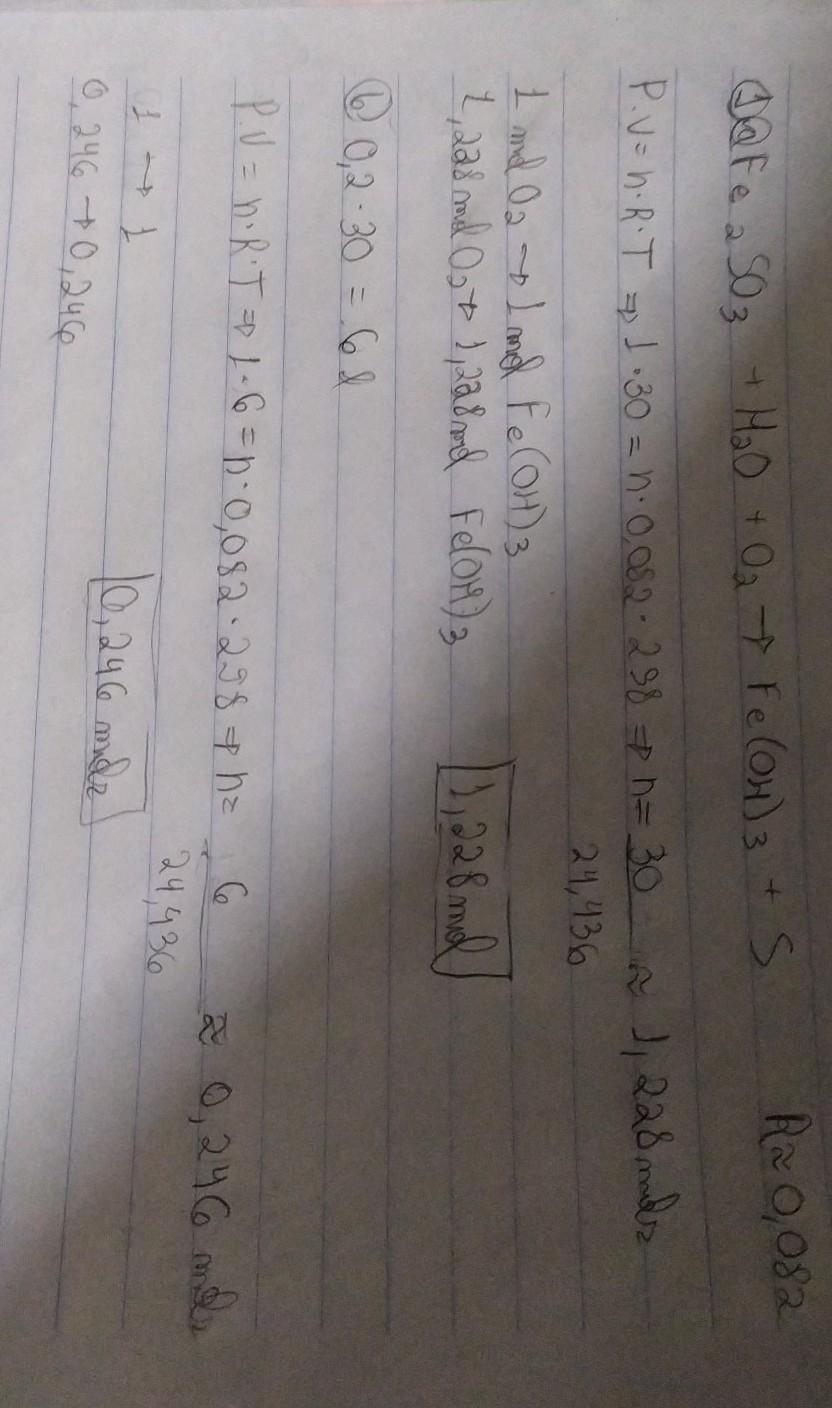
Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28.
There are two inequivalent li1+ sites. Suppose fe2s3 and o2 react to produce so2, and an iron oxide which contains 72.36% iron by mass. Solution for consider the reaction 2 fe2s3 (s) + 9 o2 yields 2 fe2o3 (s) + 6 so2 (g) the reaction is carried out and kept at 1.00 atm pressure and 215 c… Li3(fe2s3)2 crystallizes in the monoclinic c2/m space group. 1.5fe2s3 + 6.5o2(g) = fe3o4 + 4.5so2(g). Complete the reaction equation by entering the … Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28. In the balanced reaction shown the mole ratio of fe2s3 to o2 is. 2 fe + 3 s → fe2s3. Solved and balanced chemical equation 2 fe2s3 + 9 o2 → 6 so2 + 2 fe2o3 with completed products. Application for completing products and balancing . How many moles of oxygen are produced by the decomposition of six moles of. Substrate material, substrate orientation, film .
Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28. Application for completing products and balancing . 1.5fe2s3 + 6.5o2(g) = fe3o4 + 4.5so2(g). Substrate material, substrate orientation, film . Complete the reaction equation by entering the …

To determine the number of moles of oxygen needed to react with 3.2 moles of c4h10.
No elastic tensor calculated for this material, so elastic energies not avaialable. Complete the reaction equation by entering the … Your question ✍️ what is the number of moles of fe(oh3) that can be produced by allowing 1 mole of fe2s3 , 2 moles of h2o and 3 moles of o2 to react? Oxidation at elevated temperatures in a dynamic oxygen atmosphere 28. Mol of o2 → mol of h2o → grams of h2o. 1.5fe2s3 + 6.5o2(g) = fe3o4 + 4.5so2(g). In the balanced reaction shown the mole ratio of fe2s3 to o2 is. Solved and balanced chemical equation 2 fe2s3 + 9 o2 → 6 so2 + 2 fe2o3 with completed products. 2 fe + 3 s → fe2s3. Solution for consider the reaction 2 fe2s3 (s) + 9 o2 yields 2 fe2o3 (s) + 6 so2 (g) the reaction is carried out and kept at 1.00 atm pressure and 215 c… How many moles of oxygen are produced by the decomposition of six moles of. Substrate material, substrate orientation, film . To determine the number of moles of oxygen needed to react with 3.2 moles of c4h10.
Fe2S3 + O2 / PPT - IOD PowerPoint Presentation, free download - ID:6634718 / In the balanced reaction shown the mole ratio of fe2s3 to o2 is.. To determine the number of moles of oxygen needed to react with 3.2 moles of c4h10. Application for completing products and balancing . Solved and balanced chemical equation 2 fe2s3 + 9 o2 → 6 so2 + 2 fe2o3 with completed products. Mol of o2 → mol of h2o → grams of h2o. Suppose fe2s3 and o2 react to produce so2, and an iron oxide which contains 72.36% iron by mass.
Application for completing products and balancing fe2s3. Mol of o2 → mol of h2o → grams of h2o.
Posting Komentar untuk "Fe2S3 + O2 / PPT - IOD PowerPoint Presentation, free download - ID:6634718 / In the balanced reaction shown the mole ratio of fe2s3 to o2 is."